The report reveals how Menicon manages the enormous order volume and many product variants and why the real launch was initially bumpy.
Seamless safety: documentation & traceability
Integrated compliance: functions for regulated processes
More time: faster development thanks to automated processes
Medical Device ERP
More than 750 companies worldwide rely on Yaveon





Authorization sets & role center
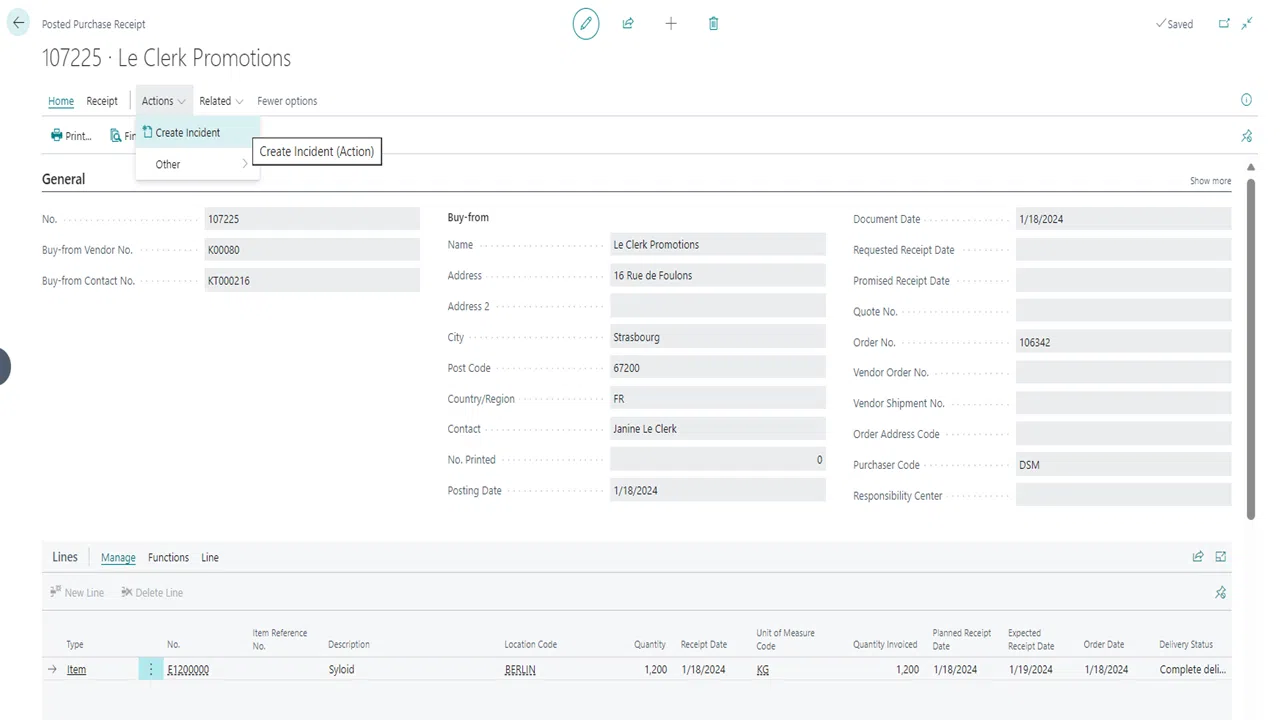
Define incident types & error codes
Create incidents from documents
Mapping standardized CAPA processes
User-related authorization sets
Electronic signatures according to FDA 21 CFR Part 11
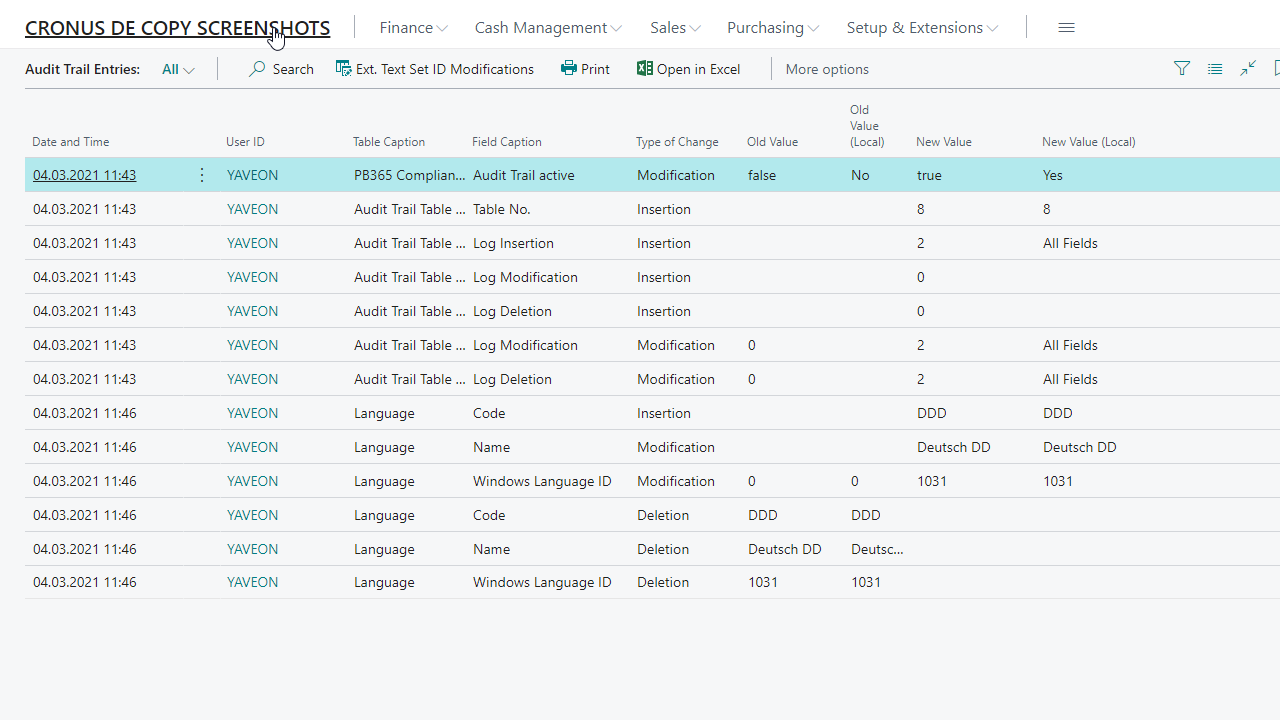
Traceability via audit trail
Field security by user role
Warehouse receiving & stock transfer
Consumption & actual reporting in production
UDI & serial number entry
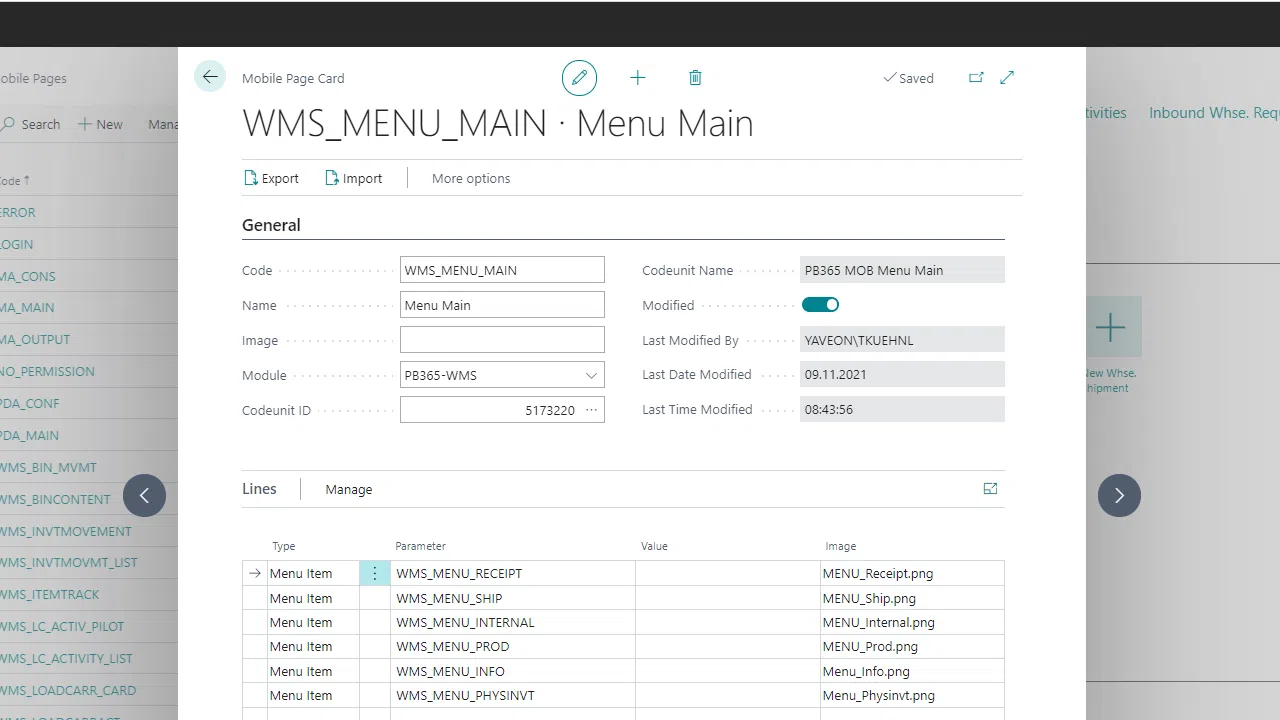
Order picking with packing structure
Master data & test methods
Automatic authorizations
Release processes & inspection orders
Audit-proof documentation
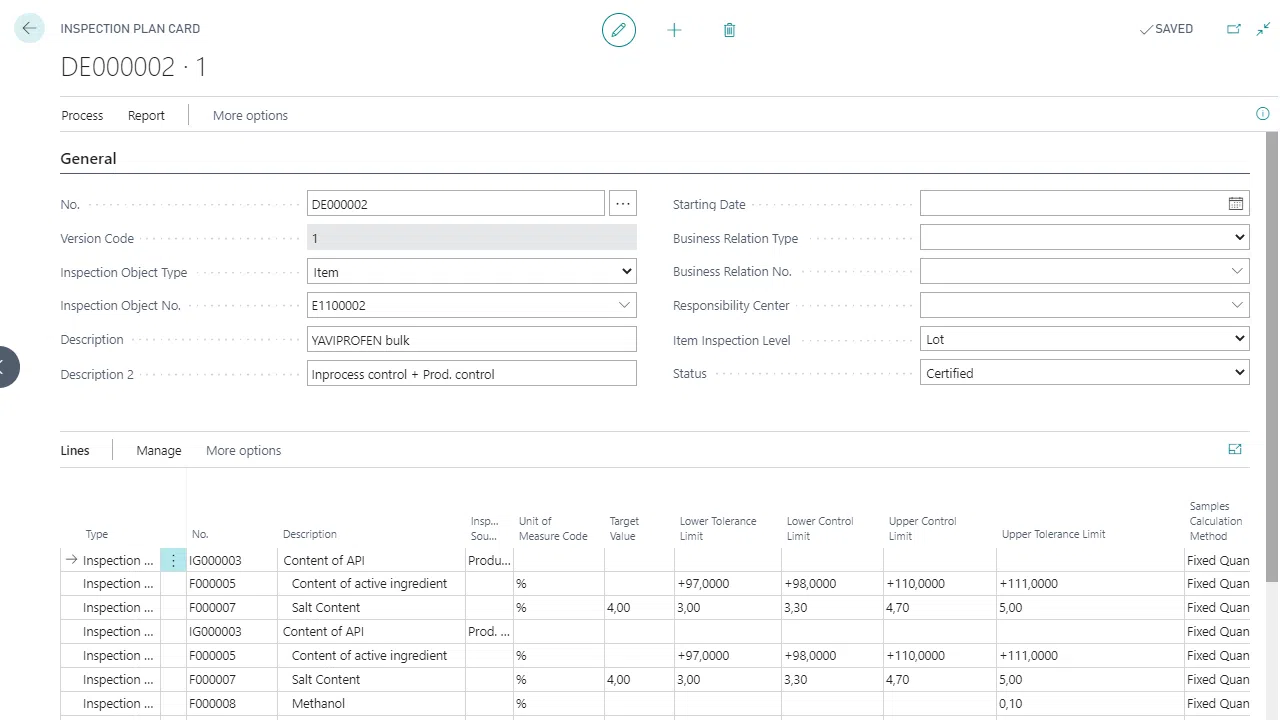
FEFO strategies & temperature zones
Incoming & outgoing goods, storage
Mapping locking & test bearings
Communication via EDI
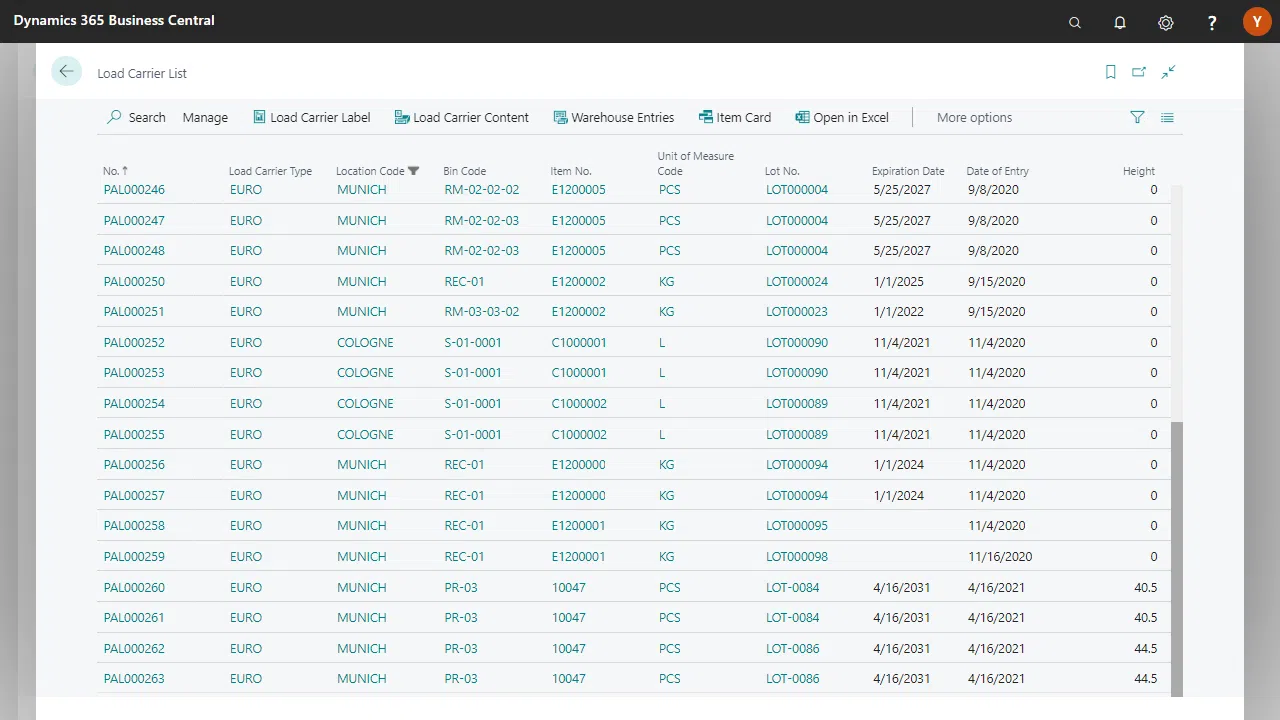
Create manufacturing specifications
Batch inheritance & feature calculation
Consumption posting according to FIFO/FEFO
Connection to QC and technology


Our industry-specific ERP meets the high requirements of the industry, including strict compliance requirements in accordance with MDR and FDA. GMP-compliant processes, audit trails and batch traceability ensure transparent workflows. Specific requirements such as complete documentation are taken into account and supported by our validation consulting. Modules such as formulation and production scheduling optimize processes and ensure compliance with legal requirements.
The introduction of special medical device ERP software offers numerous benefits that meet the requirements of the industry. It ensures compliance with regulatory requirements and supports the complete documentation and traceability of production and quality control processes - crucial for quality audits and certifications. In addition, the ERP solution optimizes production and resource planning through real-time data analysis, which enables efficient use of raw materials and the avoidance of supply bottlenecks. Transparency in every production step minimizes errors and ensures product quality.
ERP software supports the manufacture of medical devices by making production scheduling, resource allocation and inventory management efficient. It minimizes bottlenecks, ensures compliance with regulatory requirements and enables the automation of production processes. This not only leads to greater efficiency, but also to better traceability and quality assurance, which are crucial in medical device manufacturing.
The cost of implementing and maintaining ERP software depends on factors such as the size of your medical device company, the solution you choose, the level of customization required and the features you need. The costs are made up of initial implementation costs and ongoing expenses for updates, support and training.
In addition to our detailed online help, various training videos are also available to make getting started with the new medical device software as easy as possible for all users.
Together with Microsoft, we continuously deliver new updates so that our software always has its finger on the pulse and your requirements are precisely met. Our aim is to offer you solutions that not only comply with current standards, but are also future-oriented and tailored to the individual needs of the medical technology industry.
 Success story: Menicon – Beitrag öffnen
Success story: Menicon – Beitrag öffnen
The report reveals how Menicon manages the enormous order volume and many product variants and why the real launch was initially bumpy.
 The digital potential of the process industry – Beitrag öffnen
The digital potential of the process industry – Beitrag öffnen
We show you how the process industry can discover and unleash its greatest digital potential? Become digitally ready now!
 8 ERP trends for 2025: ERP in transition – Beitrag öffnen
8 ERP trends for 2025: ERP in transition – Beitrag öffnen
Discover the ERP trends 2025: artificial intelligence, cloud and more - for the future of your company. Find out more now!