Find out how you can regulate audit trails & electronic signatures, comply with FDA 21 CFR Part 11 & revolutionize your pharmaceutical release processes with the ERP industry solution.
Reduced complexity: for more flexibility
Audit-safe processes: complies with essential regulations
Controlled Supply Chain: seamless oversight and insights








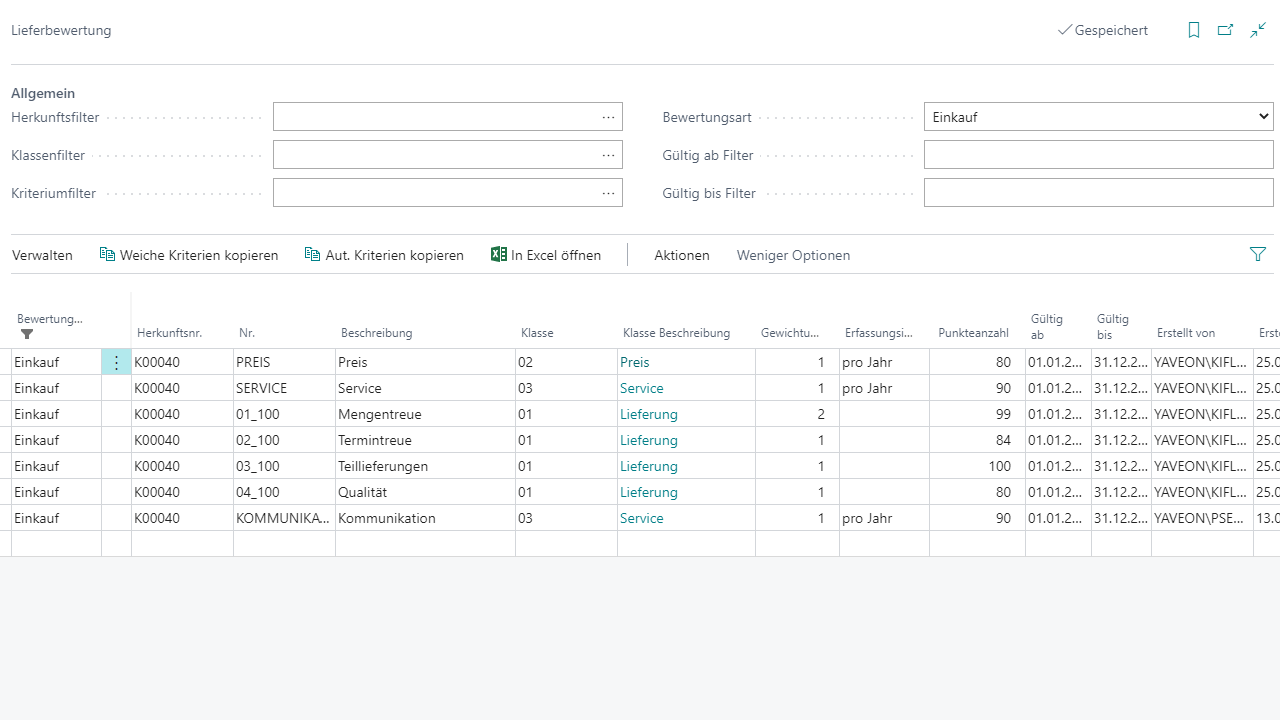
Internal & External Supplier Evaluation
Assigning evaluation criteria
Deadline reliability, quantity reliability & partial shipments
Creating reports based on automated criteria
Assigning permission sets
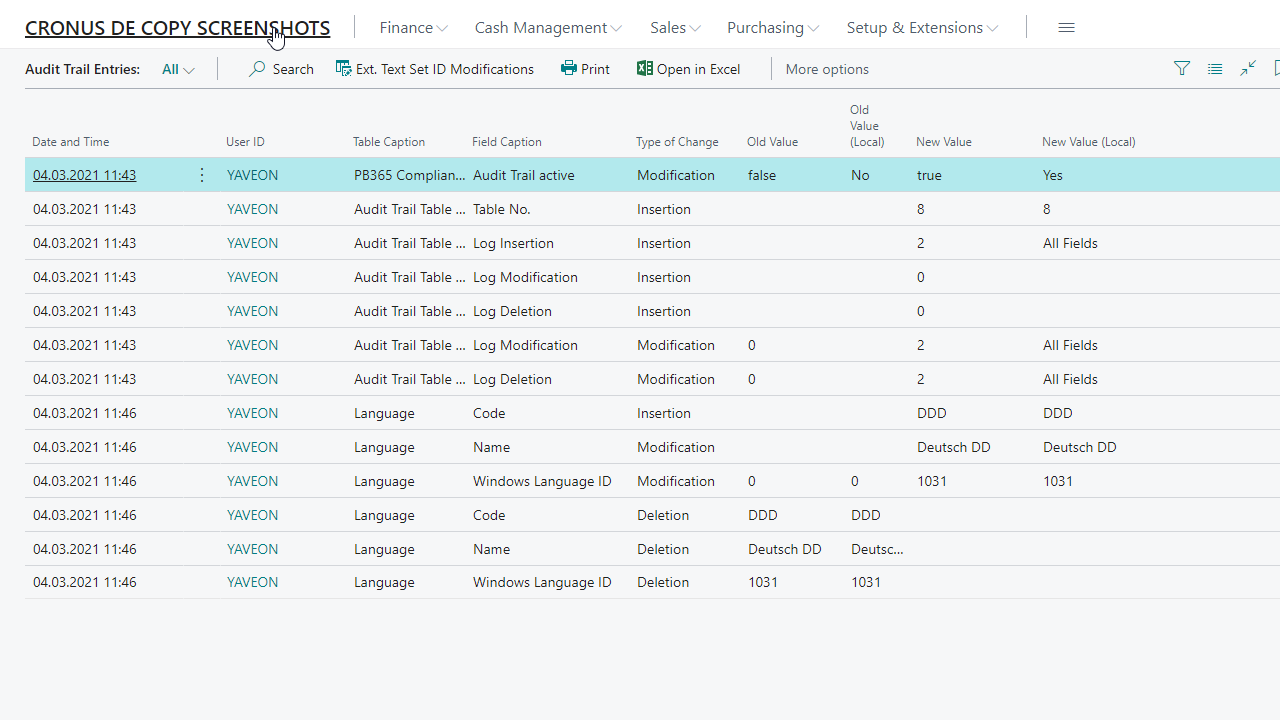
Electronic signature
Tracking of database changes with the audit trail
Field security for user-dependent rights
Warehouse receiving dialogues
Transfer orders
Report actual consumption and status for production
Order picking dialog in warehouse shipping, including packing structure
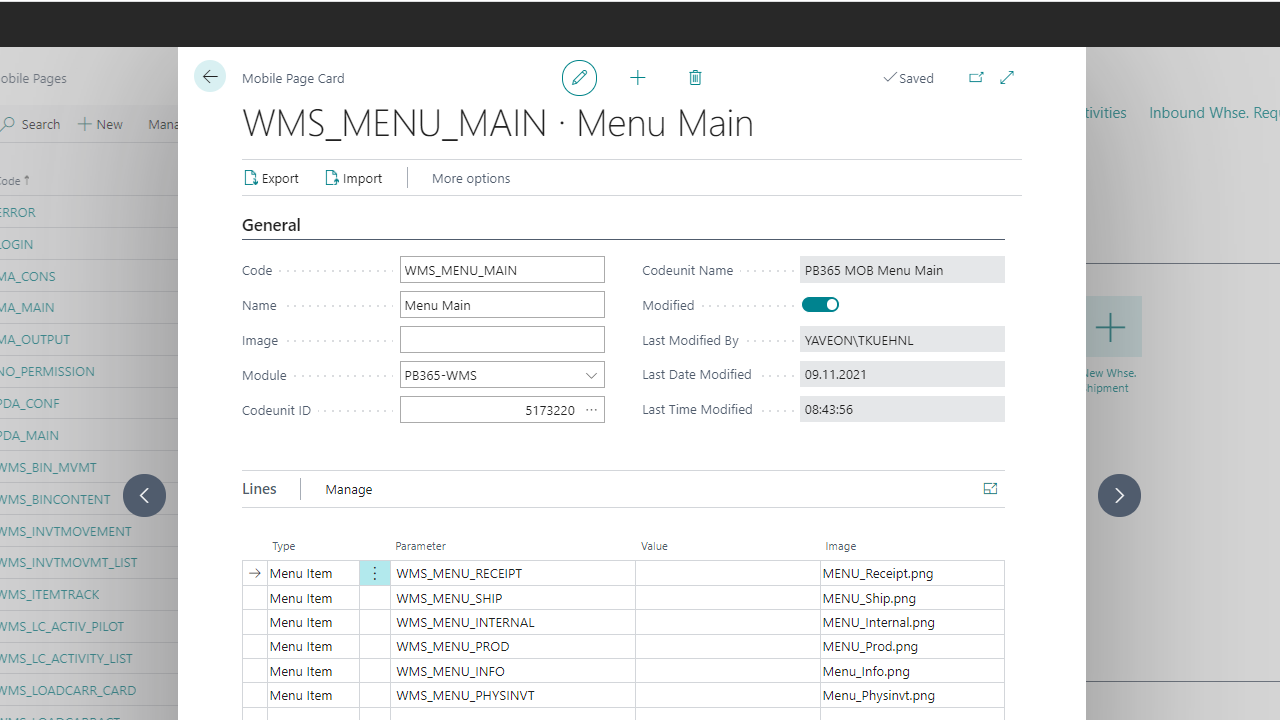
Setting up master data
Automatic provisioning of permission sets
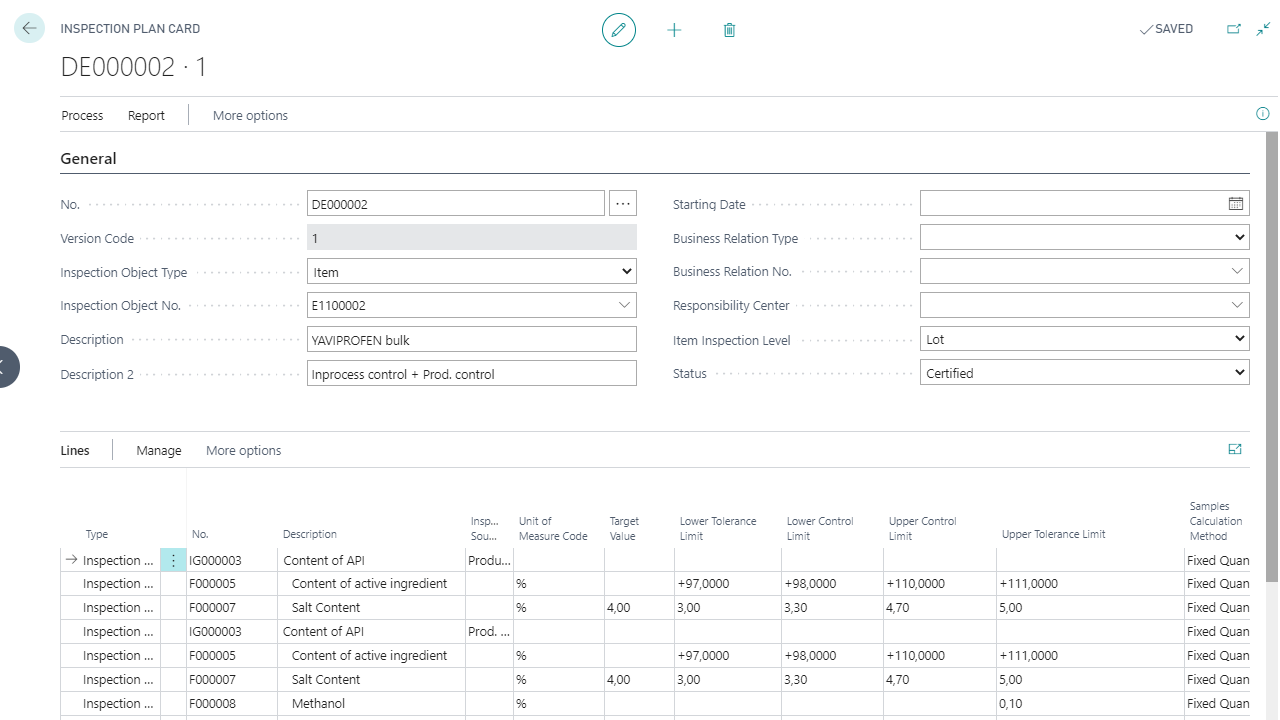
Stability test orders & retention sample management
Definition and execution of inspection orders
Initialization of the inventory list
Automatic installation of permission sets
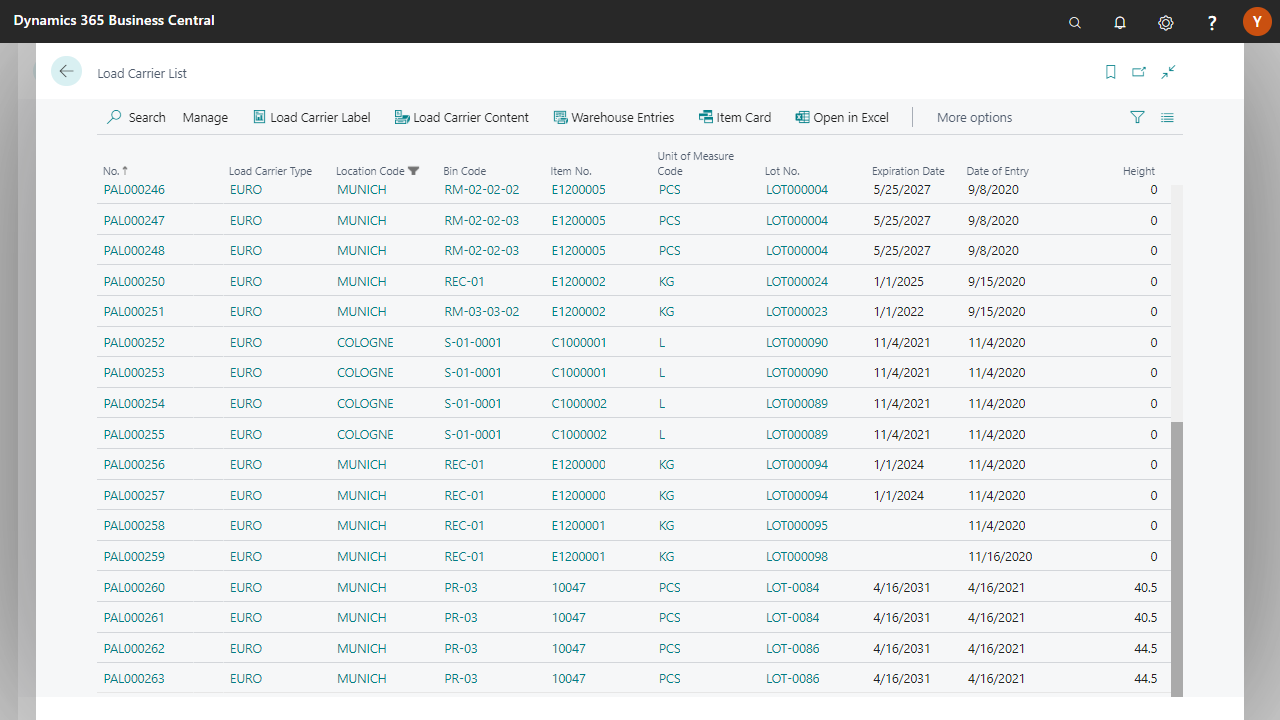
Creation of warehouse receiving, storage, and shipment
Definition of the pick order
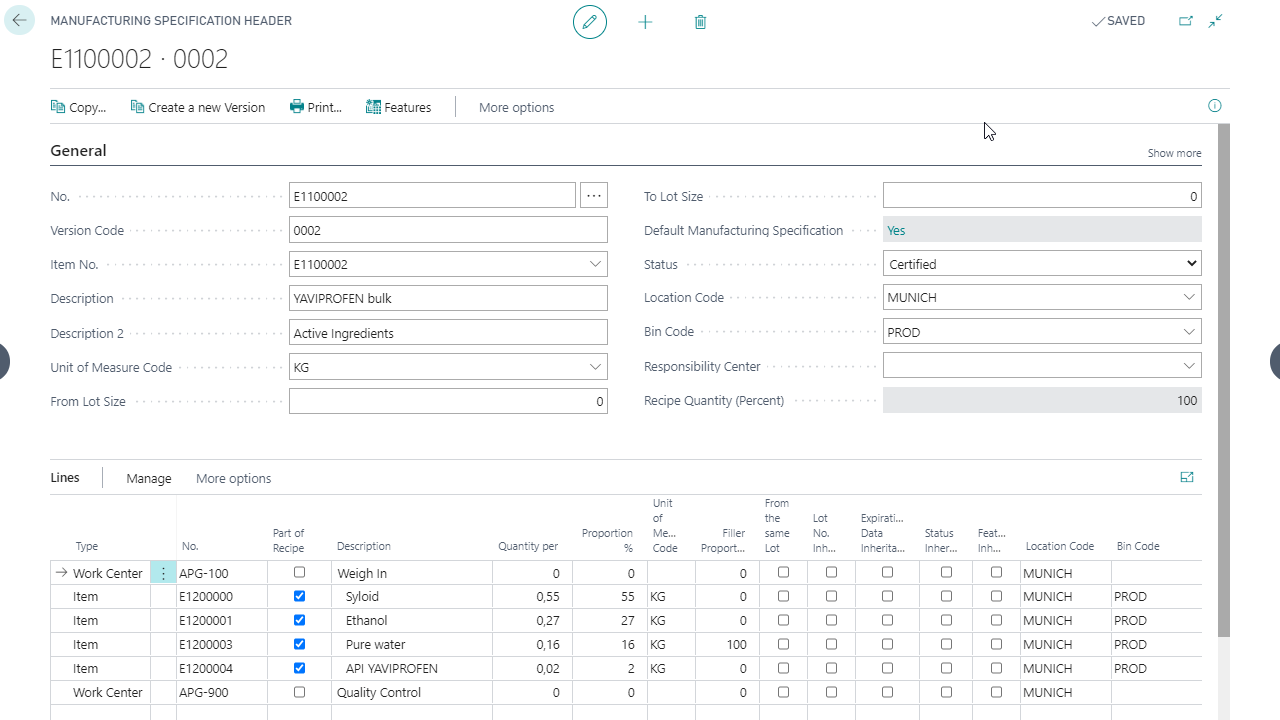
Creating manufacturing instructions
Batch inheritance in production
Consumption recording according to FIFO/FEFO
Multilevel feature calculation
Setting up item tracing
Management of batches and serial numbers (Traceability)
Rule management for product labels with posting rule groups
Multilevel batch release


Implementing ERP software in the pharmaceutical industry ensures compliance with GMP and FDA standards, guarantees traceability, and offers transparency. Production processes are optimized, resources are utilized more efficiently, costs are reduced, and automation minimizes errors. Integrated recipe management shortens development times and speeds up market entry. Companies gain efficiency and enhance their competitiveness.
With integrated features like comprehensive audit trails and seamless lot tracking, pharmaceutical ERP software ensures transparent and accurately documented processes. Additionally, validation consulting provides targeted support for creating essential documentation and processes to efficiently meet regulatory requirements. Specialized modules, such as those for formula and production scheduling, not only optimize internal operations but also ensure compliance with legal regulations throughout the entire manufacturing process.
Implementing an ERP system in the pharmaceutical industry requires precision and industry-specific expertise. Key challenges include regulatory requirements, system validation, and the integration of features such as lot tracking and quality control. With Microsoft Business Central and Yaveon 365, we offer a specialized solution. Our project methodology, ProCedures, ensures a structured and efficient implementation process. We place great emphasis on the secure migration of your data to ensure a seamless transition.
The costs for implementing and maintaining an ERP system vary based on the size of your pharmaceutical company, the chosen software solution, the degree of customization, and the required features. Total costs consist of one-time implementation expenses and ongoing costs for updates, support, and training. Detailed cost planning helps you allocate your budget efficiently and consider all necessary requirements.
Yes. In addition to our extensive online help, we offer a variety of training videos to ensure that all users can easily get started with the new pharmaceutical software. This ensures your team can work efficiently and seamlessly with the system.
Yes, absolutely. Together with Microsoft, we deliver continuous updates to ensure our software remains cutting-edge and meets your exact requirements. Our goal is to provide you with solutions that not only adhere to current standards but are also future-oriented and tailored to the unique needs of the pharmaceutical industry.
 Audit trail & electronic signatures – Beitrag öffnen
Audit trail & electronic signatures – Beitrag öffnen
Find out how you can regulate audit trails & electronic signatures, comply with FDA 21 CFR Part 11 & revolutionize your pharmaceutical release processes with the ERP industry solution.
 Success story: Hameln Pharma – Beitrag öffnen
Success story: Hameln Pharma – Beitrag öffnen
Read how hameln pharma connects an external warehouse largely with a standard interface and saves time through automated processes.
 Success story: Midas Pharma – Beitrag öffnen
Success story: Midas Pharma – Beitrag öffnen
Read the report to find out how Midas uses a direct warehouse connection to the external logistics provider and processes orders automatically.