Optimise your food industry supply chain: boost efficiency and margins with the right ERP system.
Full traceability: Across all batches and stages
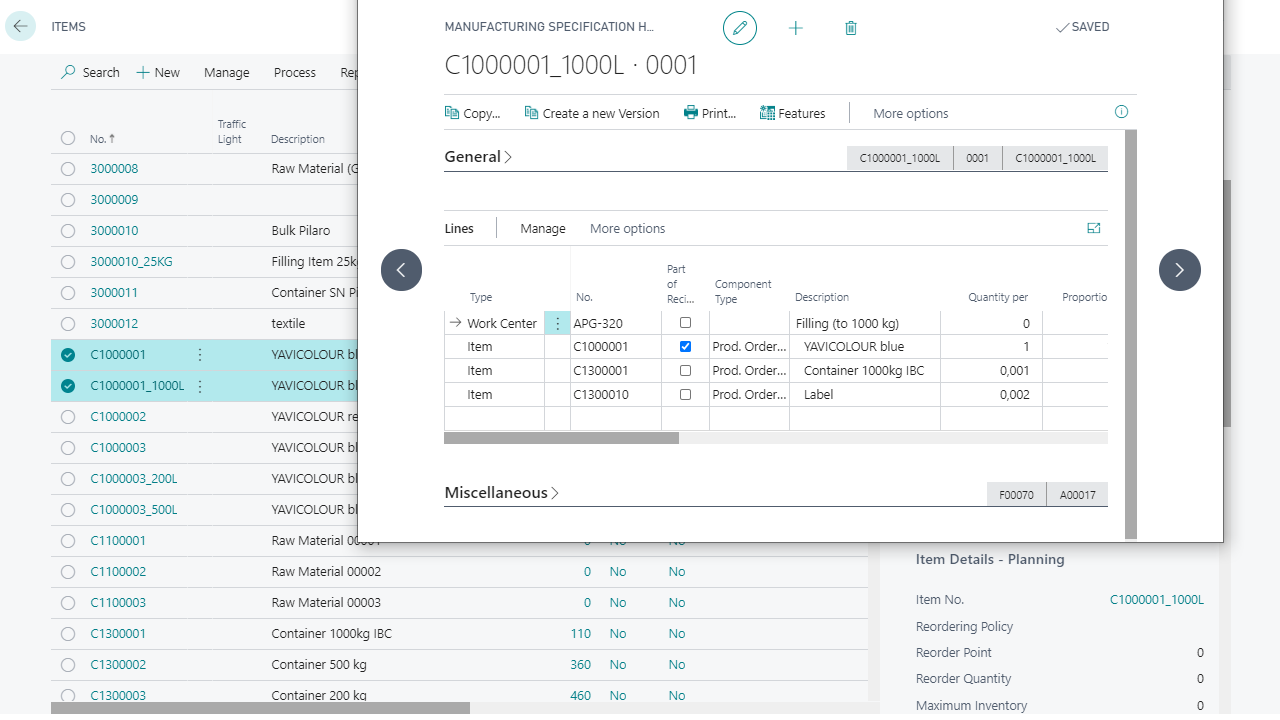
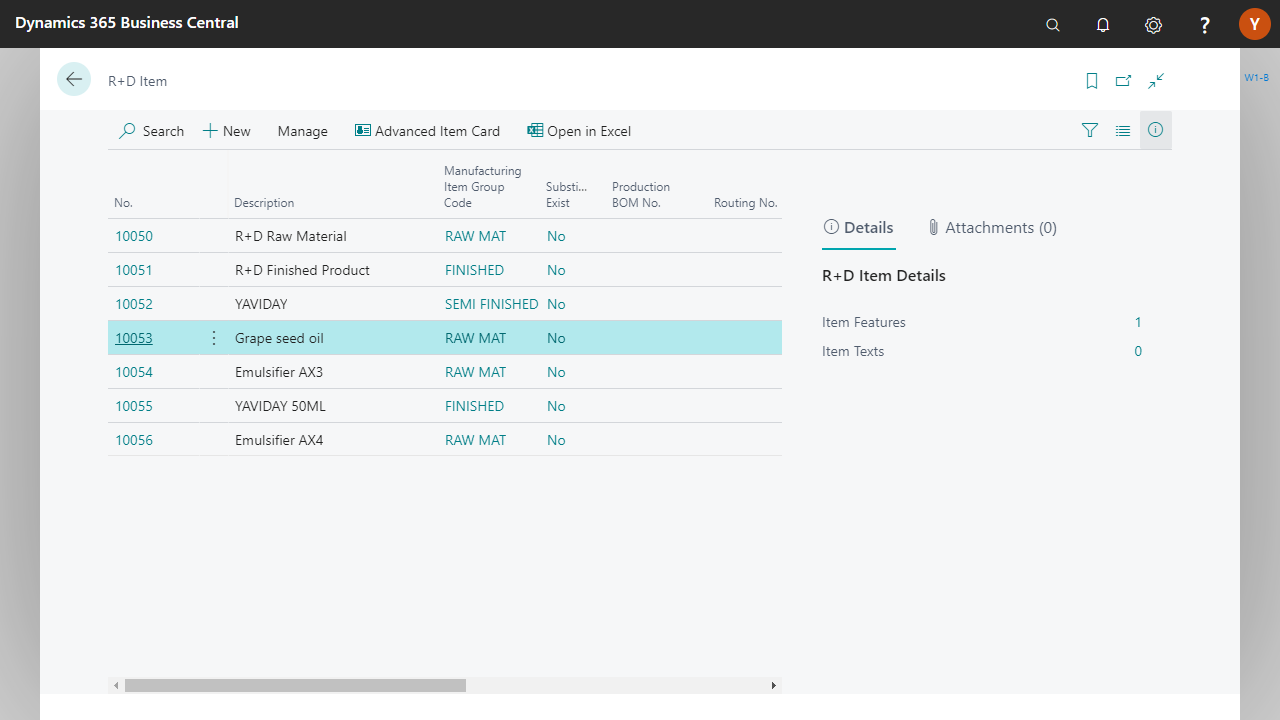
More innovation: integrated formulation management
Highest compliance: Meets HACCP & GMP requirements







Consider versions, bulk density & bulk volume
Target value/potency adjustment based on raw material characteristics
“What-if” calculations & scenario comparison
Full transparency of weighings in one system
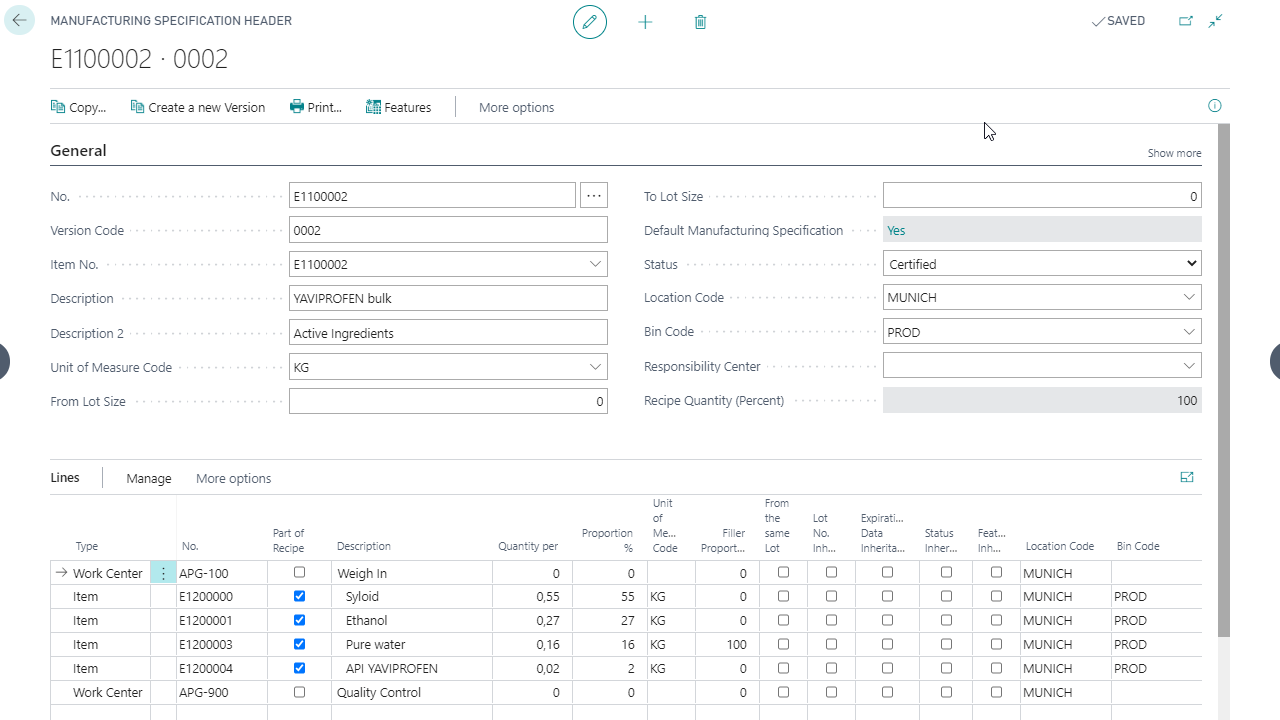
Automatically generate weighing orders during the production process
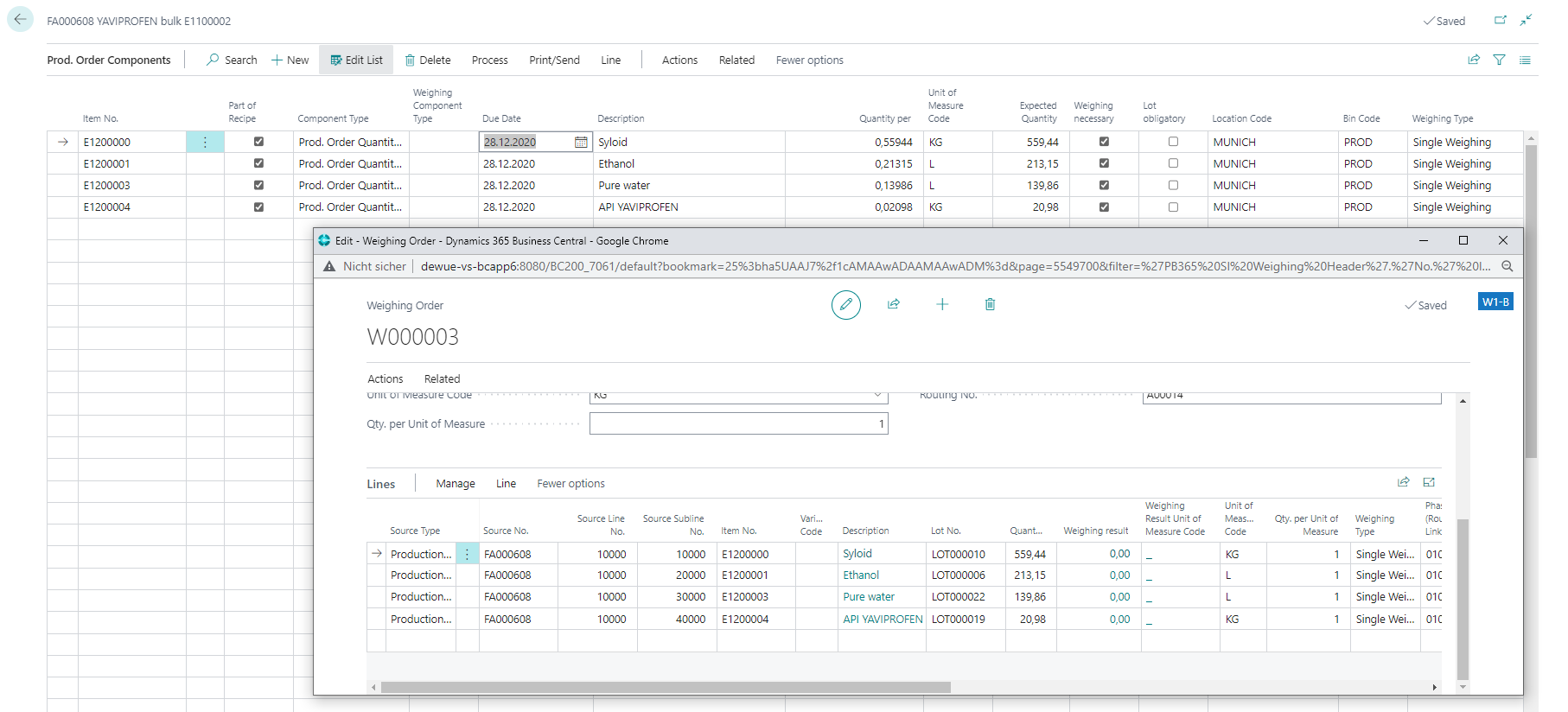
Transferring the weighing position to the weighing system
Simple consumption and actual reports
Full transparency of weighings in a single system
Multistage process chains
Dynamic formulation adjustment for pH, starch, and moisture
Feedback including scrap, yield, and OEE metrics
Traceability down to partial containers
Inspection plans for warehouse receiving, IPC, and final inspection
Deviation, complaint, and CAPA processes
COA per batch, including potency, hardness, and disintegration
Supplier evaluation through inspection and certificate data
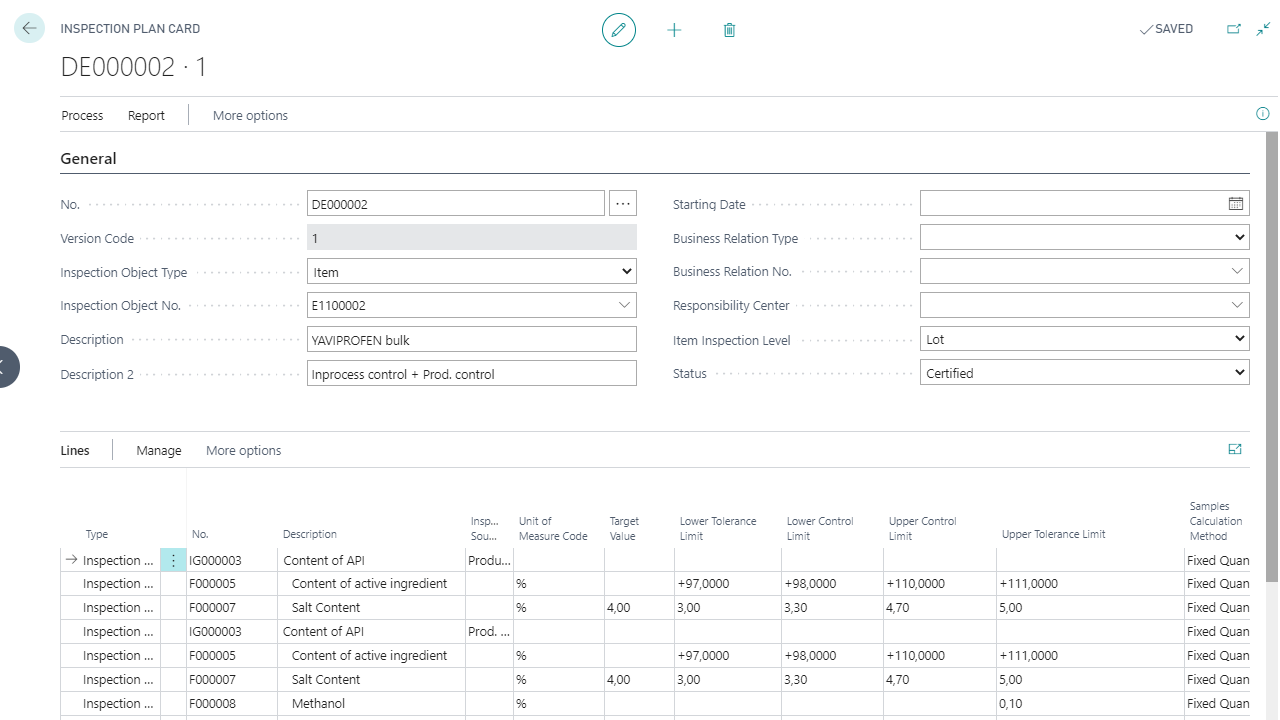
Documentation of hazard analyses & CCPs (HACCP)
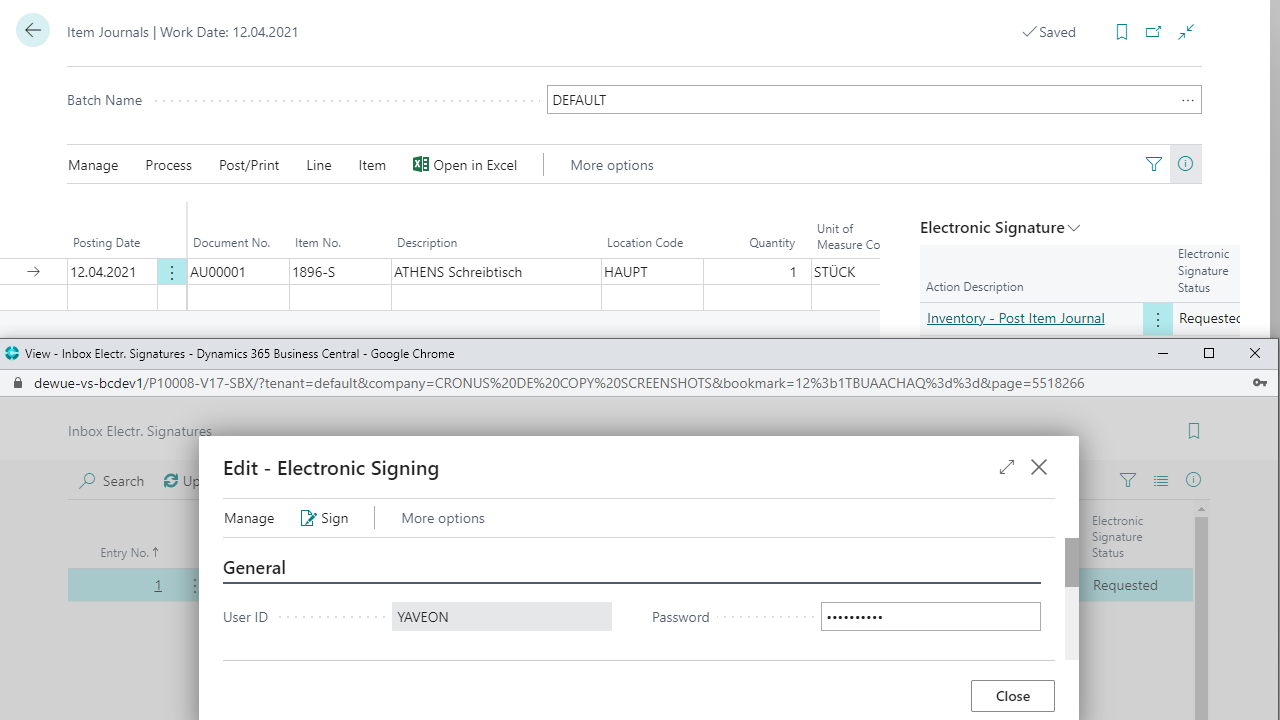
Electronic signatures, versioning & locking mechanisms
Validated process steps and test plan verifications
Mock recall & test reports at the push of a button
Automatic nutrient and active ingredient calculation per portion
Health claim-compliant data provision
Batch and best-before date labeling down to the packaging level
GS1-/GFSI-compliant accompanying documents
FEFO/FIFO/LIFO order picking per item
Automatic best-before and expiration warnings
Catch-weight management for variable containers
SSCC/GS1 support for pallets and shipping
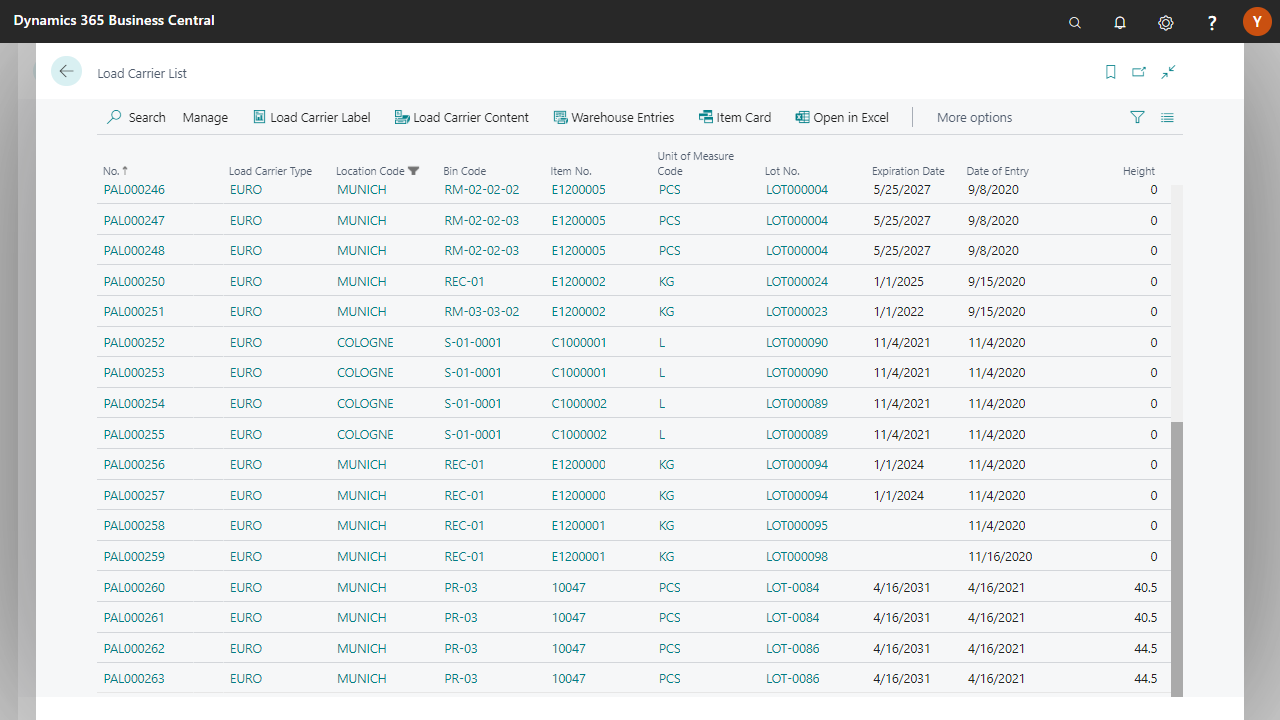

Yaveon 365 digitally documents every batch. From warehouse receiving to shipment, the solution automatically records every movement and change. Electronic batch records (eMBRs), audit trails, and release protocols ensure complete documentation. The ERP automatically generates recall reports.
Yaveon 365 centrally maintains formulations, including precise active ingredient and nutritional value calculations. Changes or new versions are controlled during approval, while labels automatically adopt the current declaration data.
Yaveon 365 supports GMP-compliant work through electronic inspection plans, digital signatures, and traceable approvals. The solution systematically identifies and assesses deviations or OOS cases (out of specification) and documents them via CAPA workflows.
Whether private label or own brand: our industry-specific ERP maps multi-level production processes, including customer-specific recipe packages and documents. Variants and approvals remain clearly separated and are traceable at all times.
The ERP automatically matches certificates of analysis (COAs) with the stored specifications. Audit results, inspection reports, and certificates are included in the supplier evaluation and determine the respective approval status.
Stability data and shelf-life rules automatically govern disposition and release processes. A batch is only released once all inspections are complete and the documentation criteria are fully met—completely digital and audit-proof.
 Supply chain management food industry – Beitrag öffnen
Supply chain management food industry – Beitrag öffnen
Optimise your food industry supply chain: boost efficiency and margins with the right ERP system.
 Quality control in food production – Beitrag öffnen
Quality control in food production – Beitrag öffnen
See how quality control keeps food safe and consistent through testing and regulatory standards.
 How to trace food and beverages safely – Beitrag öffnen
How to trace food and beverages safely – Beitrag öffnen
Traceability means being able to track food throughout its entire life cycle. We explain how you can guarantee this.